Charge Transfer Drives Hydrogen Adsorption, Spillover, and Hydroxylation
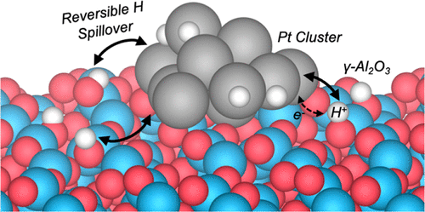
Metal−support interactions have garnered much attention due to their impact on the structure and reactivity of supported metal catalysts. Despite the widespread recognition of multifunctional mechanisms in metal / metal oxide systems, much less attention has been paid to how the metal influences its support. Here, metal−support interactions are explored usinghydrogen adsorption on a dehydroxylated γ-Al2O3(110) supported Pt10 cluster as a prototype. Through molecular dynamics simulations performed using an actively trained machine-learned force field, reversible hydrogen spillover was observed between the support and the metal. Analysis of the electronic structure and chemical nature of the interface reveals that charge transfer from H to the Pt10 cluster drives the spillover by stabilizing H adsorbed on the support. The same charge transfer concept also explains the stabilization of OH fragments at the Pt10/γ-Al2O3(110) interface despite the scarcely impacted or even reduced acidity of interfacial Al sites. These findings demonstrate the rich chemistry of metal−support interfaces and the importance of “inverse” effects in the fundamental understanding of supported catalysts.