Investigation of Carbonation Kinetics in Carbonated Cementitious Materials by Reactive Molecular Dynamics
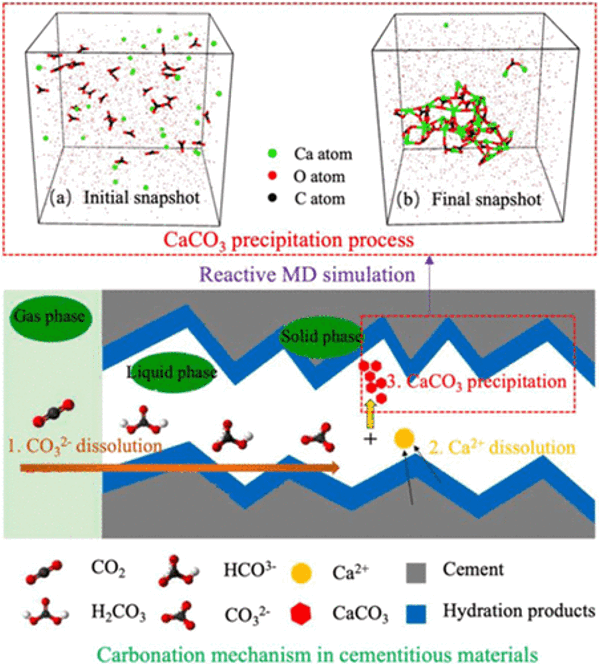
Cement concrete is a calcium-containing material, so it can capture and store carbon dioxide itself mainly via three ways: 1) carbonating the raw materials of cement concrete before concrete preparation, 2) carbonating the fresh concrete during the mixing period of raw materials, and 3) accelerated carbonation curing of cement concrete. Direct carbonation of recycled aggregate can improve its density, reduce water absorption, and improve mechanical properties. Among these three methods, carbonation curing has the highest carbon sequestration efficiency, which is about 3−4 times that of the other two methods. Calcium carbonate (CaCO3) precipitation plays a significant role during the carbon capture process; however, the mechanism is still only partially understood. Understanding the atomic-level carbonation mechanism of cementitious materials can promote the mineralization capture, immobilization, and utilization of carbon dioxide, as well as the improvement of carbonated cementitious materials’ performance. Therefore, based on molecular dynamics simulations, the effect of Si/Al concentrations in cementitious materials on carbonation kinetics has been investigated. Results show that the Ca–Oc bond number and carbonate cluster size increase with the decrease of the Si/Al concentration and the increase of temperature, leading to the higher amorphous calcium carbonate gel polymerization degree. The local stress of each atom in the system is the driving force of the gelation transition. The presence of Si and Al components increases the atom’s local stress and average charge, thus causing the increase of the energy barrier of CaCO3 polymerization and the activation energy of carbonation.