Enhancing the Electrocatalytic Activity of Molybdenum Disulfide
Mar 9, 2021
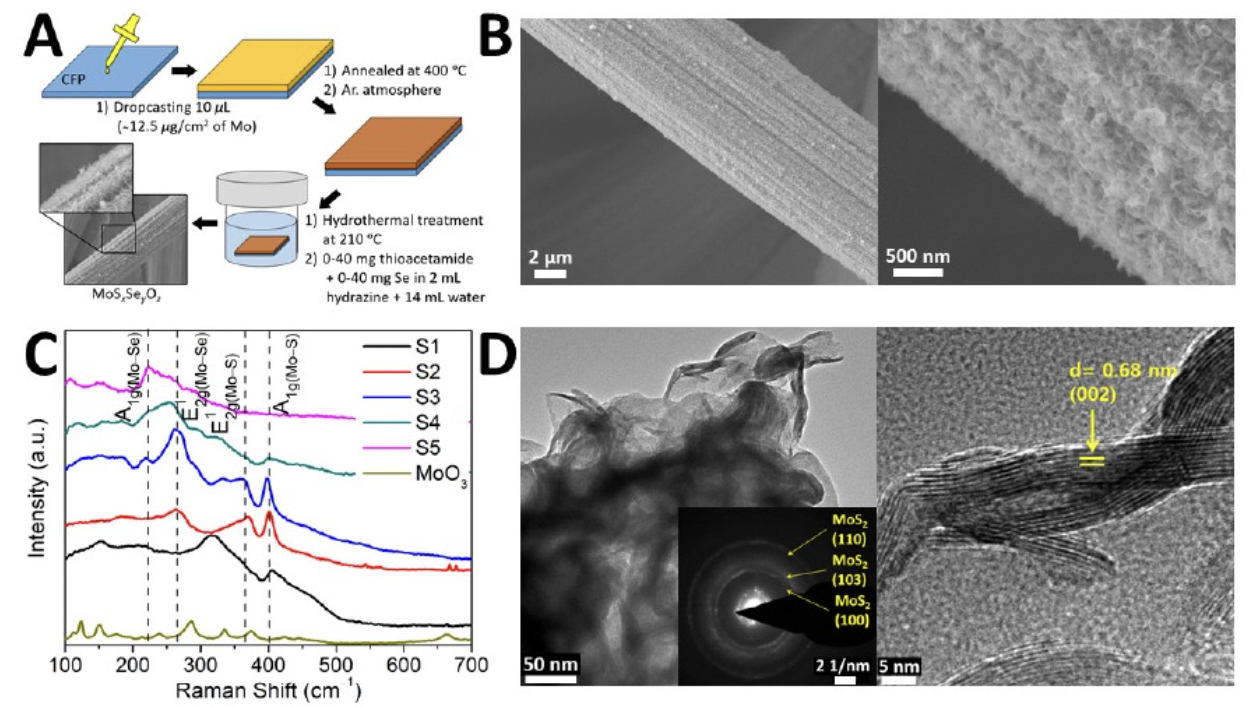
Electrocatalytic water splitting is a promising approach to harness the power of hydrogen, a clean fuel that can be burnt in air to release energy and produce only water as the byproduct. Most catalysts for the hydrogen and oxygen evolution reactions, such as precious metals, are expensive, scarce, and difficult to source. Parija et al. investigated selenium substitution on the anion sublattice and interfacial hybridization with molybdenum trioxide (MoO3) to enhance the catalytic activity of molybdenum disulfide (MoS2). The resulting MoS 2-xSex/MoO3 heterostructures demonstrate several improvements, including low overpotentials, high current densities, high turnover frequencies, and prolonged operation. This modification scheme came about as a result of closed loop X-ray absorption and emission spectroscopy measurements of electronic structure, as well as density functional theory calculations. These materials showed excellent electrocatalytic activity for hydrogen and oxygen evolution reactions. The work highlights the potential of modulating crystal and electronic band structure to enhance the performance of inexpensive, Earth-abundant alternatives for electrocatalytic water splitting.
Publication
Authors
Sarbajit Banerjee