Melting temperature of silicate T/EBCs
Rare-earth silicates are the current standard material for use as environmental barrier coatings for SiC-based ceramic matrix composites as hot-section components in gas-turbine engines. Expanding the design space to all available rare-earth elements to facilitate optimizing functionality requires an understanding of systematic trends in RE2Si2O7 properties. In this work, we combine first-principles calculations with experimental measurements of Young’s modulus, coefficient of thermal expansion, and thermal conductivity for a range of different RE2Si2O7 compositions and phases.
Elizabeth Opila, University of Virginia
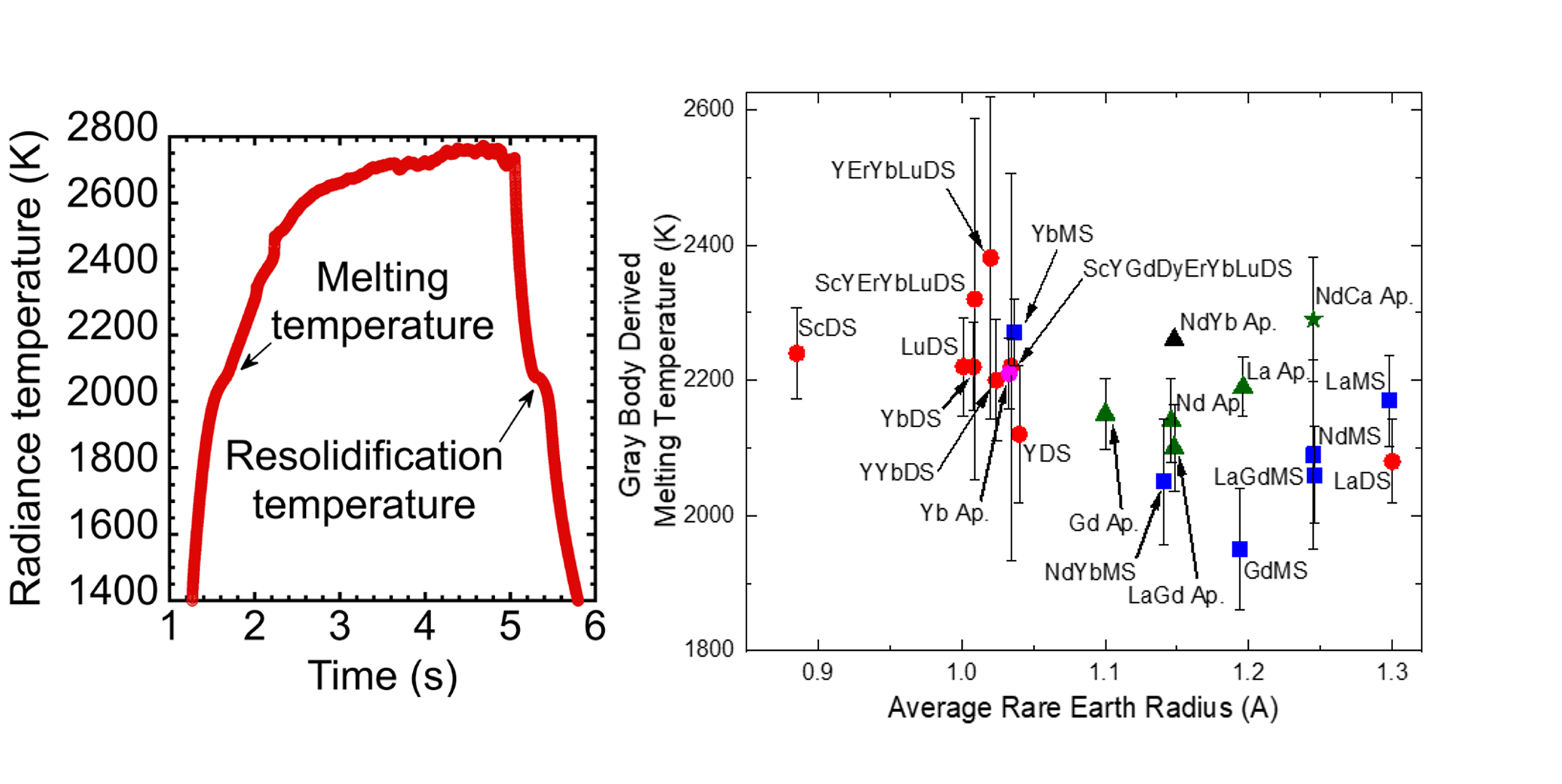
-We use a laser heating and radiation pyrometry based technique to measure melting temperature and spectral emissivity of a variety of single and multi-rare earth cation mono- and disilicates. In this technique a high power > kW continuous wave laser is used to heat the sample surface while an array of pyrometers monitor surface radiance.
-An example heating profile and resulting thermogram is shown in the left figure. We measure the melting temperature using the temperature of the thermal arrest associated with resolidification upon cooling.
-As shown on the right figure, the single-component disilicates were measured to have melting points between 2000-2250 K, while the monosilicates exhibited a wide range in melting points from 1950-2300 K. For silicates of the same rare earth element, we find that the monosilicatehas a higher melting point than the disilicate, as evidenced by the La and Yb silicates. In general, the apatite samples demonstrated even higher melting points than their monosilicatecounterparts, with Yb apatite being the exception. Yb apatite however, exhibited a second thermal arrest at ~2150 K. The four- and five-component rare earth disilicates are observed to have exceptionally high melting temperatures, more than 100 K higher than the measured single-component silicates.